Numerical Simulations of Gaseous Detonations
Multi-component Euler Equations
Reaction Terms of Detailed Chemistry
The reaction terms for detailed chemcial reaction are determined by a reaction mechanism of M chemcial reactions, i.e.
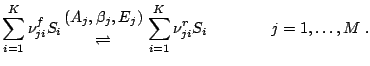
![\dot \omega_i = \sum_{j=1}^M (\nu_{ji}^r-\nu_{ji}^f)\biggl[ k_j^f\prod_{n=1}^K\Bigl({\rho_n \over W_n}\Bigr)^{\nu_{jn}^f} - k_j^r\prod_{n=1}^K\Bigl({\rho_n\over W_n} \Bigr)^{\nu_{jn}^r} \bigg] \quad i=1,\dots,K\;,](http://www.vtf.website/asc/wiki/pub/Amroc/ReactionDetailedChemistry/2c981b8b770a380f2147a51b44f6a6c9.gif)
-- RalfDeiterding - 14 Dec 2004